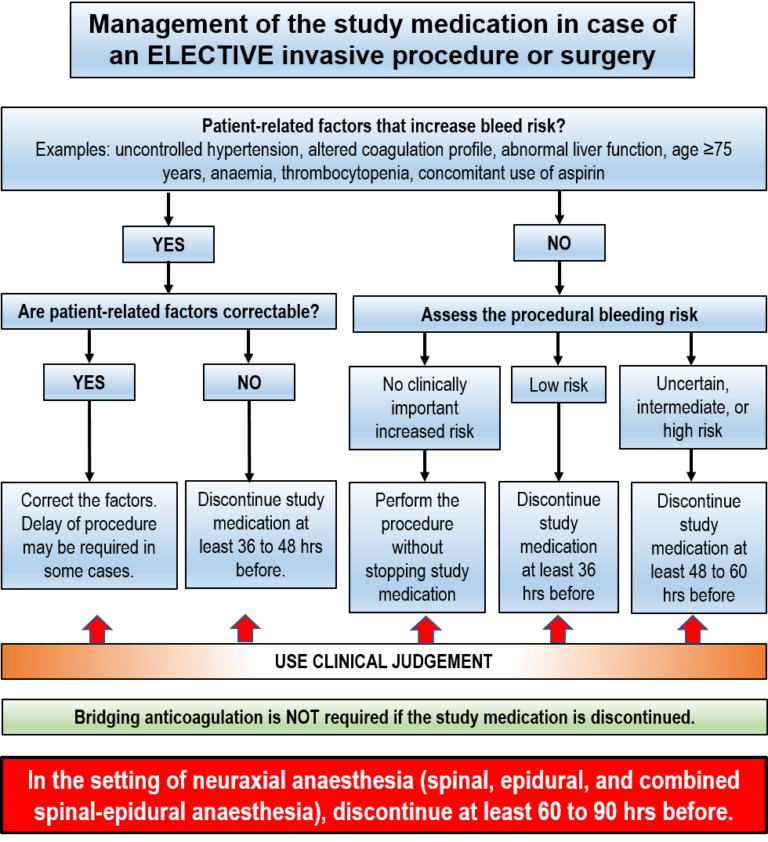
General considerations for participants requiring an invasive procedure or surgery:
- Consider whether patient-related factors that impart increased bleed risk are present. For example, uncontrolled hypertension, altered coagulation profile, abnormal liver function, age ≥75 years, anaemia, thrombocytopenia, and concomitant use of aspirin.
- Assess the bleeding risk associated with the procedure:
i. no clinically important bleed risk;
ii. low procedural bleed risk;
iii. uncertain procedural bleed risk; or
iv. intermediate/high procedural bleed risk.
*A list of common procedures and associated procedural bleed risk can be found in this document. The proceduralist’s opinion of bleed risk may vary from that proposed in this document
3.Consider the clinical effect of bleeding should it occur;
4. Whether the participant requires neuraxial anaesthesia, and
5. If the invasive procedure is an elective or emergency procedure.
Participants with patient-related bleed risk
If patient-related factors that increase bleed risk are present, correct these factors before the procedure. In some cases, electively scheduled procedures may need to be delayed, if possible, to correct patient-related factors.
If patient-related factors are not correctable, stop the study medication 36 to 48 hours before the invasive procedure or surgery or as dictated by clinical judgment.
Participants without patient-related bleed risk
In participants without patient-related factors, assess procedural bleed risk.
No clinically important bleed risk: The procedure may be performed without interrupting the study medication. Where possible, time the procedure to coincide with the trough level of the study medication (e.g. during the late morning with the prior evening dose given and a missed morning dose).
Low procedural bleed risk: The study medication should be stopped at least 36 hours before the invasive procedure or surgery.
Uncertain, intermediate or high procedural bleed risk: The study medication should be stopped at least 48 to 60 hours before the invasive procedure or surgery.
Click here for Restarting the study medication after an invasive procedure or surgery.
Click here for Restarting the study medication after neuraxial anaesthesia.