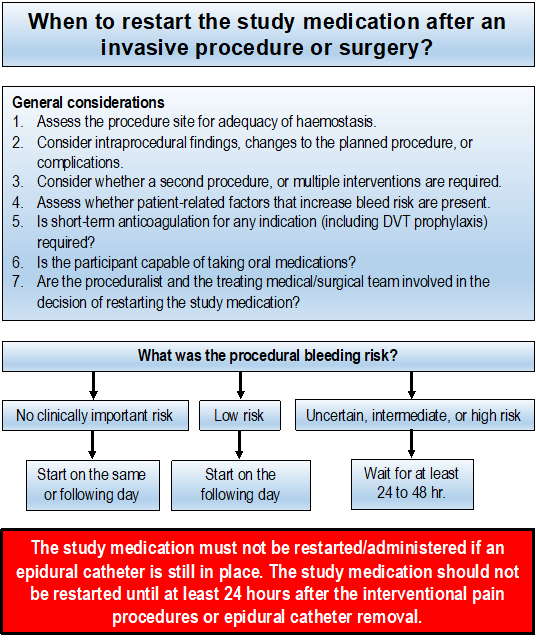
General considerations for restarting the study medication post-procedure:
- Carefully assess the procedure site to determine adequacy of haemostasis.
- Consider intraprocedural findings, changes to the planned procedure, or complications.
- Consider whether a second procedure, or multiple interventions are required.
- Consider patient-related factors that impart increased bleed risk are present. For example, uncontrolled hypertension, altered coagulation profile, abnormal liver function, anaemia, thrombocytopenia.
- Whether the participant has/is receiving short-term anticoagulation for any indication (including DVT prophylaxis).
- Consider whether the participant is capable of taking medications orally after the procedure.
- The proceduralist and the treating medical/surgical team have been involved in the decision of restarting the study medication.
No clinically important bleed risk: The study medication can be restarted in the evening on the same day if the procedure is performed in the morning. If the procedure is performed later in the day, restart the study medication on the following day morning.
Low procedural bleed risk: The study medication can be restarted on the following day.
Uncertain, intermediate or high procedural bleed risk: Wait for at least 24 hours before restarting the study medication.