|
TRACK Study
Update from The George Institute for Global Health
|
|
|
|
Issue 20 – 29th Feb 2024 Study Overview The TRACK trial will evaluate
whether a small dose of rivaroxaban, a blood-thinning medication, would
reduce cardiovascular death or major cardiovascular events in patients with
advanced stages of chronic kidney disease. |
||
|
Important
Updates & Reminders |
|
1. Words from Chief Investigator Prof. Sunil Badve
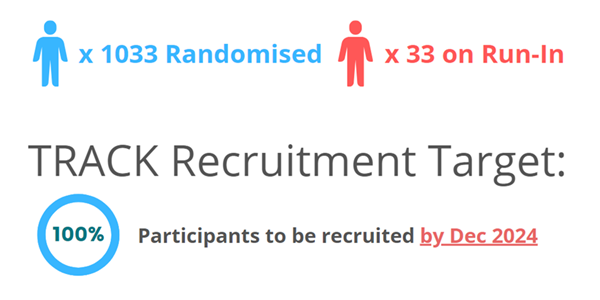
2. Recruitment The TRACK Trial passed 1000
patient randomisations shortly after the new year, reaching 1033 on
the 26th of February. It is now the largest trial to
date in cardiovascular disease (CVD) and chronic kidney disease (CKD)
populations. Another important milestone was passed recently, with our median
follow-up duration now exceeding 365 days, making TRACK the longest
follow-up trial for this cohort! Despite these impressive milestones,
we are still behind our projected recruitment rate by a significant margin.
The success of the trial is dependent on our ability to recruit and retain
patients. In order to reach our total recruitment
target of 1886 by December 2024, all countries and sites must
continue to make a significant contribution to participant numbers. Recruitment
Strategies The nature of
the patients eligible for the TRACK trial means that they are regular
visitors of clinics, hospitals and dialysis units.
Periodic review of patient lists from these locations is a good strategy for
recruiting new participants. ·
National Leads play a crucial role in setting the tone
for regional sites, providing guidance, encouragement
and advice. ·
Investigator Meetings are an excellent opportunity to
discuss recruitment strategies specific to the region and gain insights from
high recruiting sites. ·
Adress concerns patients may have regarding bleeding risk
by communicating the low rates of bleeding seen in the TRACK trial and the
stringent safety measures. 3. Bleeding Risk A reminder that in the most recent
DSMB report from December 2023 the bleeding rate for major bleeds was
reported as 3.4 per 100 person years (py).
This is significantly lower than what is reported in our Patient Information
Sheet (10.0/100py) and lower than rates seen in both dialysis and
non-dialysis patients in a recent comparison of similar trials. The
reason is likely two-fold: 1. The TRACK trial is using a low
dose a Rivaroxaban, well below the therapeutic dose. 2. The TRACK trial eligibility
criteria carefully excludes any high-risk patients. This low bleeding rate is very
encouraging and should provide reassurance to patients enrolled in the TRACK
trial. If you have any particular concerns
with regards to bleeding, please do not hesitate to reach out to our
coordinating investigator Prof. Sunil Badve directly. We encourage staff at sites to
communicate to patients that the Patient Information and Consent Form risk
rates are not reflective of those seen in our therapeutic dose. The regular
DSMB screening and stringent safety measures outlined in our study protocol
are additional points of reassurance. 4. Outcomes Vs
SAEs A recent review of Serious Adverse
Event (SAE) listings has identified some instances of misreporting. ·
Peripheral Artery Disease (PAD) events like amputations
are primary outcomes and should be reported as such, not as
SAEs. ·
Potential cardiovascular deaths, stokes and non-fatal
myocardial infarctions are all primary outcomes. We ask staff to carefully consider,
when reporting an SAE, if the event could potentially be an outcome. If you
are unsure if an event is an SAE or an outcome, please do not hesitate to
reach out to your CRA for confirmation or refer to Appendix 1 of the protocol
which provides a comprehensive overview of outcome definitions. Capturing all outcome events under
the correct categorisation is crucial, as underreporting can impact
statistical power and trial integrity. Important Reminders: ·
Good Clinical Practice Certifications
must be within 3 years for all staff. Please requalify as soon as
possible if certifications are outside this period. ·
High rates of Withdrawal Post Randomisation and Lost to
Follow-up participants have the potential to negatively impact study power. ·
High rates of IMP Discontinuation may also result
in loss of study power. This has been highlighted by the DSMB in Dec 2023.
For IMP discontinuation due to participant request (not due to adverse
event or bleeding), please keep communication open for participants to
consider resuming IMP. |
|
|
|
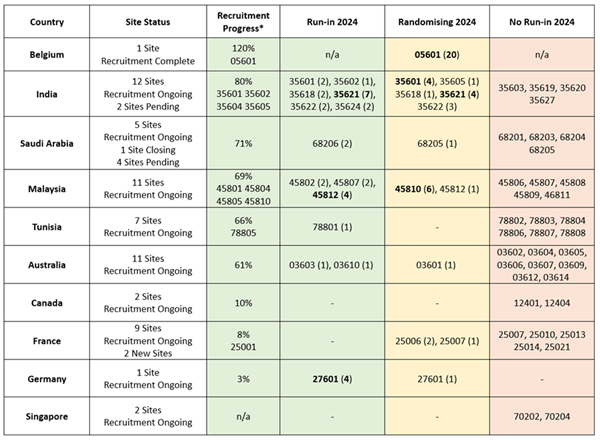
Recruitment
and Country Updates All figures are as of 26 Feb 2024
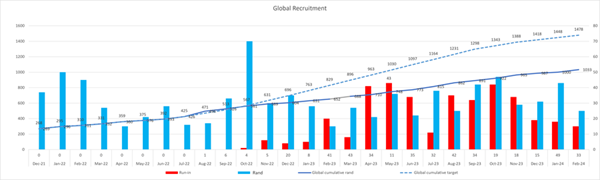
Global Recruitment Graph: Monthly Progression Below
figures are as of 26 Feb 2024
Global Recruitment Graph: Randomised vs Target Below
figures are as of 26 Feb 2024
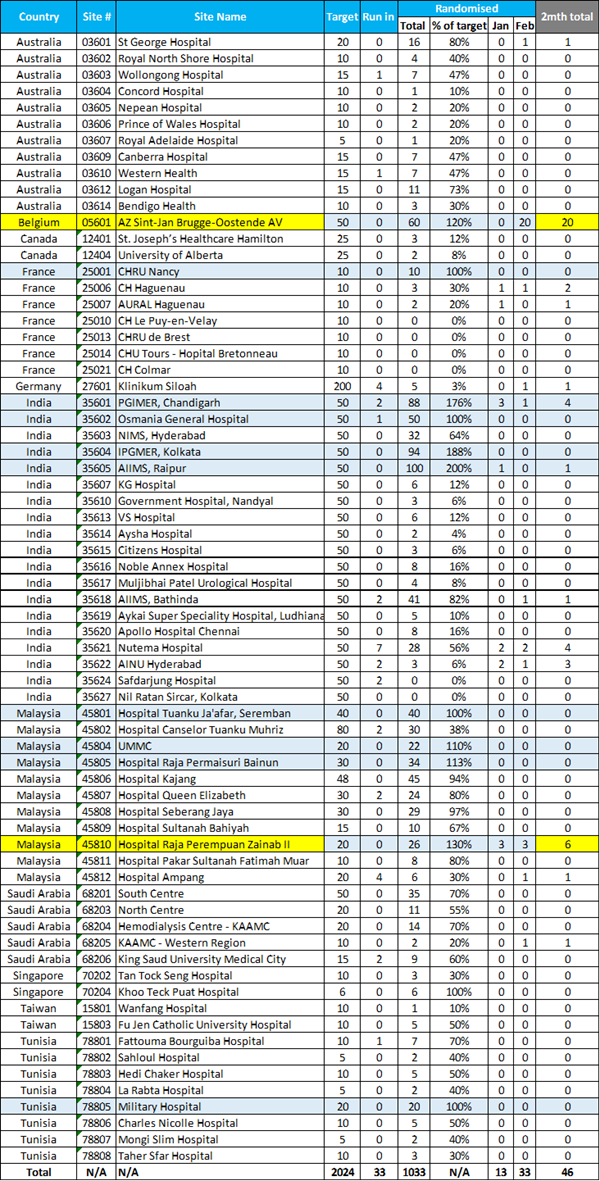
January-February Site Recruitment Blue Sites have
met or exceeded target. Yellow sites are top 2 recruiters for Jan 01-Feb 26
period.
|
|
New Sites We would like to warmly welcome the
below new sites that are now activated for the TRACK trial: Country: France Country: France We are looking forward to seeing the
first participants enrolled at your sites! |
|
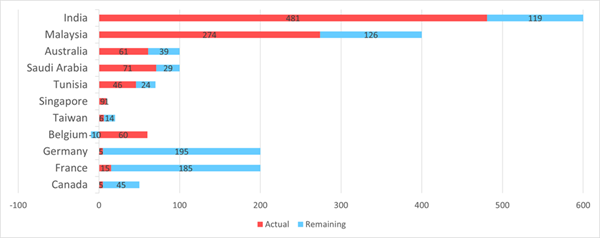
January-February Regional Recruitment *Percentage of
Recruitment target met by Feb 26 2024. Note: Taiwan
no longer recruiting.
|
|
TRACK
Feature Profile
Name: Dr. Sandeep Garg Site 35621
Nutema Hospital has randomised 28 patients since its activation on the 24th
of August 2023. They enrolled their first participant only 4 days after site
activation and have continued to perform excellently in the subsequent
months. In today’s Newsletter, Nutema is currently the joint leader of
recruitment in 2024 for India, with 4 randomisations and across the
January-February period. Our thanks go out to Dr Garg and the Nutema site
staff for their phenomenal efforts. |
|
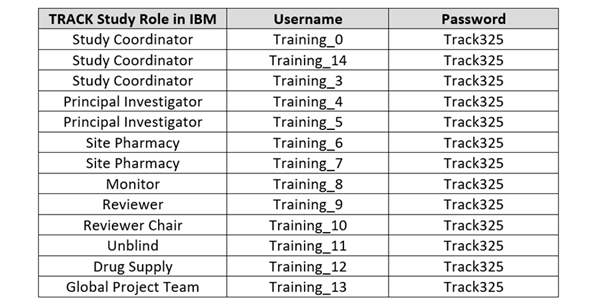
IBM Training Database Logins For any site staff who wish to train/practice
data entry for the study, please visit the TRACK Training IBM Database by
logging in with any of the following updated training accounts.
Thank you for
supporting the Track Trial. To learn more please visit www.tracktrial.org Warm regards, The TRACK Trial team |
|
|