|
|
Issue
13
| 21 December
2022
|
|
|
Study Overview
The
TRACK trial will evaluate whether a small dose of rivaroxaban, a
blood-thinning medication, would reduce cardiovascular death or major
cardiovascular events in patients with advanced stages of chronic
kidney disease.
|
|
|
|
Global Participant
Recruitment Charts
We
greatly encourage all sites to increase recruitment in the new year.
Below figures are as of 19 Dec 2022.
|
|
|
1. Reporting
of gastrointestinal bleeds
The only minor bleeding events that require reporting
are the ones that fall under the categories in TRACK Protocol Appendix
1:
- 8.1 Overt
bleeding of gastroduodenal origin confirmed by endoscopy or
radiography.
- 8.2 Overt
upper
gastrointestinal bleeding of unknown origin.
- 8.3
Bleeding of presumed occult upper
gastrointestinal tract origin with documented decrease in
haemoglobin of 20 g/L
Minor bleeds that do not fall under these
categories, such as minor lower gastrointestinal bleeding, do
not require reporting. There is a focus on the reporting of upper
gastrointestinal bleeding due to concerns regarding antithrombotic
medications causing gastric erosions/ulcers.
Any other minor bleeds do not need to be reported except for
the GI bleeds in Protocol Appendix 1.
|
|
|
2.
Undetermined cause of death
Some cardiovascular deaths have been reported as
'Undetermined Cause of Death' within the TRACK database.
The following death scenario qualifies as a cardiovascular (CV)
death and not 'undetermined cause of death':
Unwitnessed
death in a subject seen alive and clinically stable ≤24 h before
being found dead without any evidence supporting a specific non-cardiovascular
cause of death.
Information about the patient’s clinical status preceding death should
be provided if available in the ‘Briefly describe the event’ field in
the eCRF. For more information, please refer to outcome
definitions in TRACK Protocol Appendix 1.
|
|
|
3. Remote
participant visits
If a participant for any reason cannot attend a clinic
visit in-person, the visit can be conducted remotely by
phone/teleconference and study medication can be dispensed through IBM
and shipped to the participant by post.
With the latest update to Protocol version 4.0, remote consent,
screening, and randomisation visits are also possible. If your
region/site would like to undertake e-consent, please inform the TRACK
Global Project Team at least 2 months in advance to allow for set up
time.
|
|
|
1. Holiday
period and emergency unblinding
During the upcoming holiday period,
database management staff will be offline from Saturday 24th
December 2022 to Tuesday 3rd January 2023.
If for any reason your site needs to perform emergency unblinding for a
participant and the usual emergency unblinding method does not work,
please contact the emergency unblinding team at the Lyon Poisons
Centre Hotline (toll-free) on +334 72 11 69 11.
|
|
|
2. DSMB
meeting & recommendations
The recent DSMB meeting was held on 8th December 2022.
The recommendation from the DSMB was 'to continue the trial unchanged' and also encouragement for 'investigators to
redouble efforts to maintain participants on study drug who do not have
a protocol-directed reason for discontinuation. The categories of
"Participant Request" and "Other" should be
minimized in order to optimize group
separation and statistical power. The Investigators could
re-approach these participants in the coming weeks and months in an effort to re-start study drug.’
We
therefore encourage sites to explain to participants the
importance of staying on the study medication as this may reduce risk
of cardiovascular events and will help to ensure we can obtain
meaningful results from the trial.
|
|
|
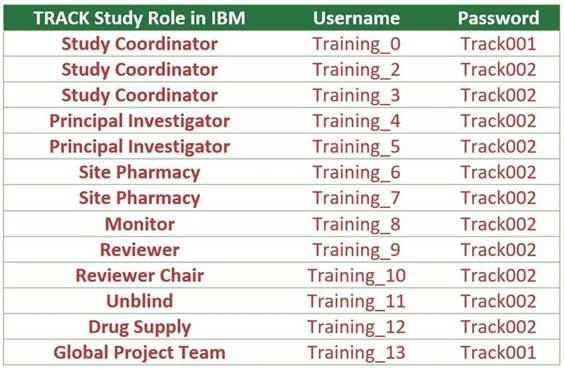
IBM Training
Database Logins
For any site staff who wish to
train/practice data entry for the study, please visit the TRACK
Training IBM Database by logging in with any of the following updated
training accounts.
|
|
|
|
|
Feature Profile
As some of you may know, our
TRACK Global Project Manager, Enmoore Lin, will be leaving the study in
the new year. From everyone in the TRACK Global Project Team, we thank
Enmoore for her unparalleled hard work and dedication to the TRACK
trial and wish her all the very best.
Below is a photo of Enmoore in her comfort zone as well as a message
from Enmoore herself.
|
|
|

|
|
Name: Enmoore Lin
Location:
Sydney,
Australia
Role: TRACK Global
Project Manager
Comment: I have had the privilege of working on the TRACK trial
from March 2020 and have been the Global Project Manager since October
2020. It has been incredibly satisfying to see the hard work pay
off and see the trial progress from start-up to recruitment in most of
our countries. There have been challenges, particularly with COVID-19
disruptions, insurmountable obstacles for China and EU regulatory
changes, but we have managed to get on top of these and I learnt a lot
in the process. Hopefully our recruitment difficulties will also fall
by the wayside. It has been a pleasure to work with everyone in
the TRACK family around the world. Thank you for your efforts, support and dedication – it has truly been a team
effort!
I will be handing over the TRACK Global Project Manager role to Jenny Landrigan on 9 January 2023. I will continue
to work on other clinical trials at The George Institute and have been
invited to join the TRACK Global Steering Committee. I look
forward to seeing the TRACK trial complete recruitment.
|
|
|
|