|
|
Study Overview
The TRACK trial will evaluate
whether a small dose of rivaroxaban, a blood-thinning medication,
would reduce cardiovascular death or major cardiovascular events in
patients with advanced stages of chronic kidney disease.
|
|
|
|
1. Expectedness for SAEs
and Outcomes
When completing the
SAE form in the database, please note that most events will be 'expected' according to the protocol or condition under
study.
If you are unsure of your response, please check with the Global
Project Team.
|
|
|
2. Asking participants
about their SAEs
During participant
phone calls and visits, please enquire about any
and all adverse events that may have occurred since their last
call/visit. If the participant confirms that an SAE occurred but it
was not treated at your hospital, please enter the event into the
TRACK database like you would normally.
|
|
|
Recruitment Graphs: Actual vs Target
The below graphs
depict the target rate of randomisation versus the actual rate
of randomisation for the TRACK trial overall and for each individual
country. Please note that only countries with
participants randomised have been included.
Below figures are as of
22 Aug 2022.
|
|
|
Study Updates & Reminders to Sites
|
|
|
1. Phosphodiesterase
Inhibitors
Please note that
the protocol lists current treatment using phosphodiesterase
inhibitors (dipyridamole) in the exclusion criteria. This has been
misinterpreted as any and all
phosphodiesterase inhibitors being prohibited with dipyridamole being
an example, however, we wish to clarify that the only
phosphodiesterase inhibitor not allowed is dipyridamole. This is updated in the FAQs page of the TRACK
website and will be clarified in the next protocol update.
|
|
|
2. Participants who
have discontinued study drug
Participants who have stopped
taking study medication should still remain
in the trial as part of follow-up and all SAEs/outcomes must continue
to be reported. Reporting timelines for 'Stopped IMP' participants
are the same as that of other participants.
|
|
|
3. Outcome & Serious
Adverse Events (SAE) that result in death
If a participant has had an
Outcome event and passes away as a result, 2 outcome forms should be
completed - one for the initial outcome and one for the death.
Similarly, if the participant has had an SAE and passes away, both
the SAE form and the Outcome form (for the death) should be
completed.
Example 1: A participant has a stroke and dies 2 weeks later. The
stroke should be entered in the Outcome form and the subsequent death
on a separate Outcome form.
Example 2: A participant is hospitalised for COVID and the event
status on the SAE form is ‘Ongoing’. If the participants later dies, the event status on SAE form should be
changed to ‘Fatal’ & an Outcome form for the death should be
completed.
|
|
|
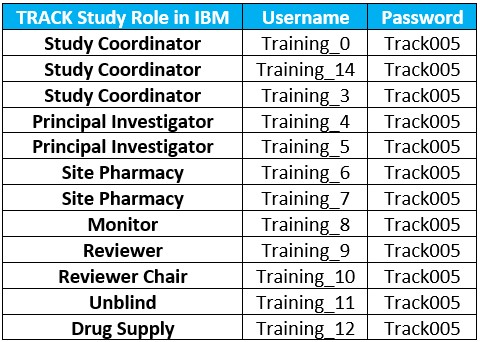
IBM
Training Database Logins
For any site staff who wish to
train/practice data entry for the study, please visit the TRACK
Training IBM Database by logging in with any of the following updated
training accounts.
|
|
|
|
|

|
|
Name: Dr. Manisha
Sahay
Position/Role: Professor and Head
of Nephrology Department
Location: Osmania General
Hospital, Hyderabad, India
About: Dr. Sahay's passion
for teaching prompted her to opt for the teaching line in the medical
profession. As a medical educator, she derives great satisfaction
from imparting training to post-graduate students in the institute.
Research forms an important part of her area of interest
and she can be attributed with numerous publications and awards
presented to her for her prolific work in her field of specialty. Her
professional areas of interest include diabetic kidney diseases and
transplantation.
In TRACK, Dr. Sahay is supported by Dr. Rakesh Sahay, Mr. Prasad, and
Ms. K. Mounika.
|
|
|

|
|
Name: Khizra
Sultana
Position/Role: Senior Clinical
Research Coordinator
Location: Riyadh, Saudi
Arabia
About: I completed my Post
graduate diploma in Clinical Pharmacy from University of Queensland,
Australia in 2007. I joined KAIMRC in 2011 & went on to complete
my Masters in Clinical Pharmacy from
University of Tasmania, Australia in 2015. I have coordinated many
national & international multicenter
clinical trials. I have been a co-investigator for a
number of projects initiated by the pharmaceutical department
at King Abdulaziz medical city. We
successfully completed & published projects on topics like
anticoagulants, tele-pharmacy, COVID-19, validation and psychometric
properties of instruments, prediction models etc. in well reputed
scientific journals. I am passionate about research & love to
give back to the research community. Presently, I am a regular peer
reviewer for the journal “Frontiers”.
|
|
|
|
|