|
|
|
|
Study Overview
The
TRACK trial will evaluate whether a small dose of rivaroxaban, a
blood-thinning medication, would reduce cardiovascular death or
major cardiovascular events in patients with advanced stages of
chronic kidney disease.
|
|
|
|
Recruitment Graphs: Actual vs Target
The below graphs depict the target rate of
randomisation versus the actual rate of randomisation for the TRACK
trial overall and for each individual country.
Please note that only countries with participants randomised have
been included.
|
|
|
Study Updates & Reminders to Sites
|
|
|
1. Hitting Site
Recruitment Target
Malaysia
Some
of our Malaysian sites have either exceeded their recruitment target
or are about to reach their target very soon, which is an impressive achievement.
We greatly appreciate the contribution and encourage all Malaysian
sites to continue recruiting at a healthy rate to help make up for
the slower recruitment of other countries.
All
other countries
We
continue to encourage all sites to increase recruitment rates and any
and all efforts to reach the trial recruitment target are greatly
appreciated.
The
current monthly global rate of recruitment of ≈25 participants
needs to increase to ≈100 participants.
To
further encourage recruitment, for sites who reach their recruitment
target and are willing to recruit beyond their target, we are open to
discussing increases in site patient payments as an added incentive.
|
|
|
2. Participant
Information and SAEs
Please
do not share any participant information (for example name, date of
birth etc.) nor the participant's SAE details to any third parties
(except regulatory authorities).
Any queries or issues regarding SAEs should be communicated directly
with the TRACK Project Team at TGI (tracktrial@georgeinstitute.org.au).
|
|
|
3. Medication
Changes
Please
remind participants to inform you or other TRACK site staff if they have
started taking any medications that they were not taking at the start
of the study.
If a participant indicates that they recently began taking a
prohibited medication, the study medication must be stopped until the
participant ceases taking the prohibited medication and the Study
Medication Log must be updated.
|
|
|
4. Patient Alert
Card
ID
Number
Please ensure that the TRACK Patient Alert Card has the participant's
study ID number written on it. In the rare occurrence whereby a participant needs to be unblinded, being
able to easily locate the participant's study ID will make the
emergency unblinding process easier for the site Investigator or
other unblinding staff involved.
Participant
Use
Please encourage participants to keep their Patient Alert Card on
them as much as possible. It has been reported that when visiting the
hospital, some participants are not able to recollect details of the
trial such as where the trial is being conducted and who is
conducting it.
|
|
|
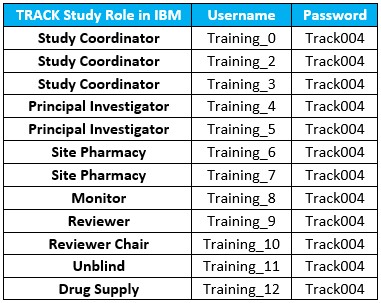
IBM Training Database
Logins
For
any site staff who wish to train/practice data entry for the study,
please visit the TRACK Training IBM Database by logging in with any
of the following updated training accounts.
|
|
|
|
|
Name: Stephanie Ren
Position/Role: Clinical Research Associate level I
Location: Singapore
About: Prior to joining George Clinical (GC), I was a
Clinical Research Coordinator in a public hospital for 2.5 years. I
had worked with both commercial and investigator-initiated trials
in the therapeutic areas of Nephrology, Endocrinology, and
Hepatology. I previously had great experiences working alongside GC
and decided to join them.
|
|
|
|
|
|
|
|