|
|
|
|
Issue 7 | 21 December
2021
|
|
|
Study Overview
The
TRACK trial will evaluate whether a small dose of rivaroxaban, a
blood-thinning medication, would reduce cardiovascular death or
major cardiovascular events in patients with advanced stages of
chronic kidney disease.
|
|
|
|
1. Two
hospitalisations for the same event
In
the situation whereby a participant has had a Serious Adverse Event
(SAE) which resulted in two (2) separate hospitalisations, these 2
hospitalisations should be reported in 2 SAE forms.
For example, a patient has an infection requiring hospitalisation on
24th May. The patient is discharged, but 6 days later is admitted to
hospital again for the same diagnosis. Both the initial
hospitalisation on 24th May and subsequent hospitalisation on 30th
May should be reported.
|
|
|
2. Outcome &
Serious Adverse Events (SAE) that result in death
If
a participant has had an Outcome event and passes away as a result, 2
outcome forms should be completed - one for the initial outcome and
one for the death. Similarly, if the participant has had an SAE and
passes away, both the SAE form and the Outcome form (for the death)
should be completed.
Example 1: A participant has a stroke and dies 2 weeks later. The
stroke should be entered in the Outcome form and the subsequent death
on a separate Outcome form.
Example 2: A participant is hospitalised for COVID and the event
status on the SAE form is ‘Ongoing’. If the participants later dies, the event status on SAE form should be
changed to ‘Fatal’ & an Outcome form for the death should be
completed.
|
|
|
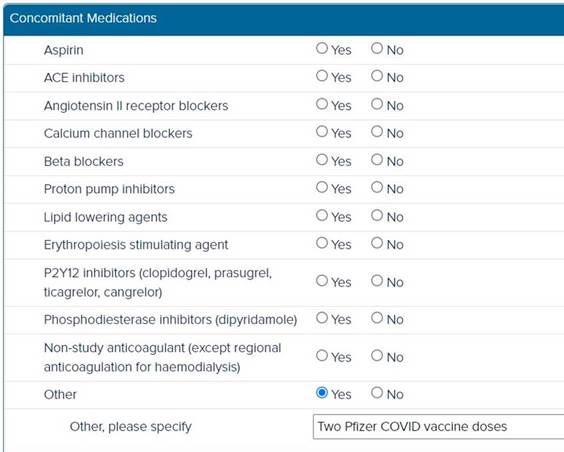
3. COVID
vaccination data entry
When
a participant receives a COVID vaccine (whether their first dose,
second dose, or booster shot), this should be entered into the
Concomitant Medications page in the eCRF.
|
|
|
|
|
4. Participants who
have discontinued study drug
If
a participant for any reason will no longer continue taking study
drug, please aim to ensure that the participant remains in the trial
as part of follow-up.
Timelines for follow-up visits, calls, and data entry should adhere
to the usual study visit windows.
|
|
|
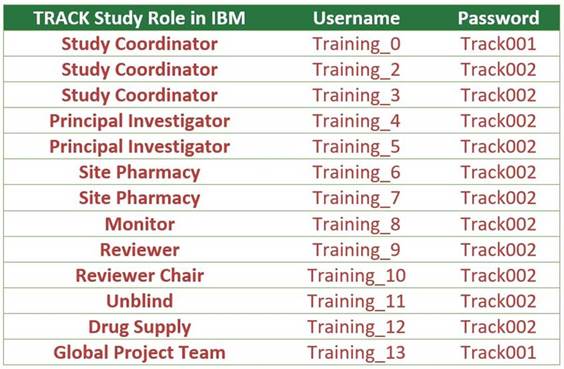
IBM Training
Database Logins
For
any site staff who wish to train/practice data entry for the study,
please visit the TRACK Training IBM Database by logging in with any
of the following updated training accounts.
|
|
|
|
|
|
|
|
|