|
|
|
|
Issue 6 | 27 October 2021
|
|
|
Study Overview
The TRACK trial will evaluate whether a
small dose of rivaroxaban, a blood-thinning medication, would
reduce cardiovascular death or major cardiovascular events in
patients with advanced stages of chronic kidney disease.
|
|
|
|
1. Missing Data in Study Medication Log
We have noticed that many sites are not
completing the Study Medical Log (SML). The SML should be completed
for any and all participants who become randomised (and therefore
taking study medication) and is not limited to stopped medications
only.
Instructions for completion:
1.The SML is to document study medication after randomisation
date ONLY. No run-in medication should be documented in the SML.
2. If study medication is actively discontinued, enter the stop date
for the current entry and then if restarted, enter a new entry in the
next row.
Note: Active discontinuation of study medication does not include
occasional missed doses. It refers to a decision made by the
participant/doctor to stop the treatment.
|
|
|
2. Extended Run-in Phase
Whilst we strongly advise that sites adhere to
the 14-30 day run-in period, we understand
that there may be exceptional circumstances whereby the site or
participant is unable to schedule or attend the Randomisation visit
within the specified visit window. In these situations, randomisation
can still occur in the IBM database, however, a protocol deviation
should be reported.
|
|
|
3. Meetings
Since the last study update, we've had the
Global Steering Committee (GSC) Meeting and Investigator Meetings
(IM) for India and Australia. We had constructive discussions and
ideas regarding participant recruitment.
India IM: Offer the TRACK trial to
every patient who comes in for a consultation. They also have the PI
available if patients have questions.
Australia IM: Aim to get recruitment momentum back once
COVID crisis eases. Would also be helpful if the PI speaks directly
to potential patients, even if they are not the PIís patient.
|
|
|
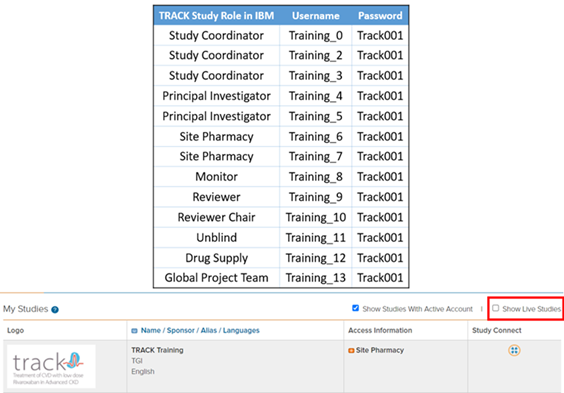
IBM Training Database
Logins
For any site staff who wish to train/practice data
entry for the study, please visit the TRACK Training IBM Database.
Reminder that upon signing in, please remember to untick the 'Show
Live Studies' checkbox so that the 'TRACK Training' database can
appear.
|
|
|
|
|

|
|
Name:
Dr. Raja Ramachandran
Role: TRACK India National Lead
Location: Chandigarh, India
About: Dr. Ramachandran
completed his DM Nephrology in PGIMER, Chandigarh. He is currently
working as Assistant Professor in Department of Nephrology PGIMER,
Chandigarh. His research specialisation includes primary glomerular
disease including adult minimal change disease, focal segmental
glomerulosclerosis, idiopathic membranous glomerulonephritis,
membranoproliferative glomerulonephritis, C3 glomerulopathy, and
atypical hemolytic syndrome.
|
|
|

|
|
Name: Dr. Sridhar Ramanaidu
Role: TRACK Principal
Investigator; Consultant Nephrologist
Location: Ipoh, Malaysia
About: Dr. Ramanaidu
joined service in the Ministry of Health Malaysia as a Medical
Officer in 2001 after completing studies in India. He graduated as an
Internal Medicine Specialist in University Malaya in 2010 and
subsequently pursued sub-specialisation in nephrology and graduated
in 2015. He has been working in Hospital Raja Permaisuri
Bainun Ipoh as a Consultant Nephrologist
ever since. His special interest is in General Nephrology and
dialysis.
"I work along with 3 other dedicated nephrologists and our good team work made us achieve high recruitment numbers
not only in this study but other ongoing studies as well."
|
|
|
|
|
|
|